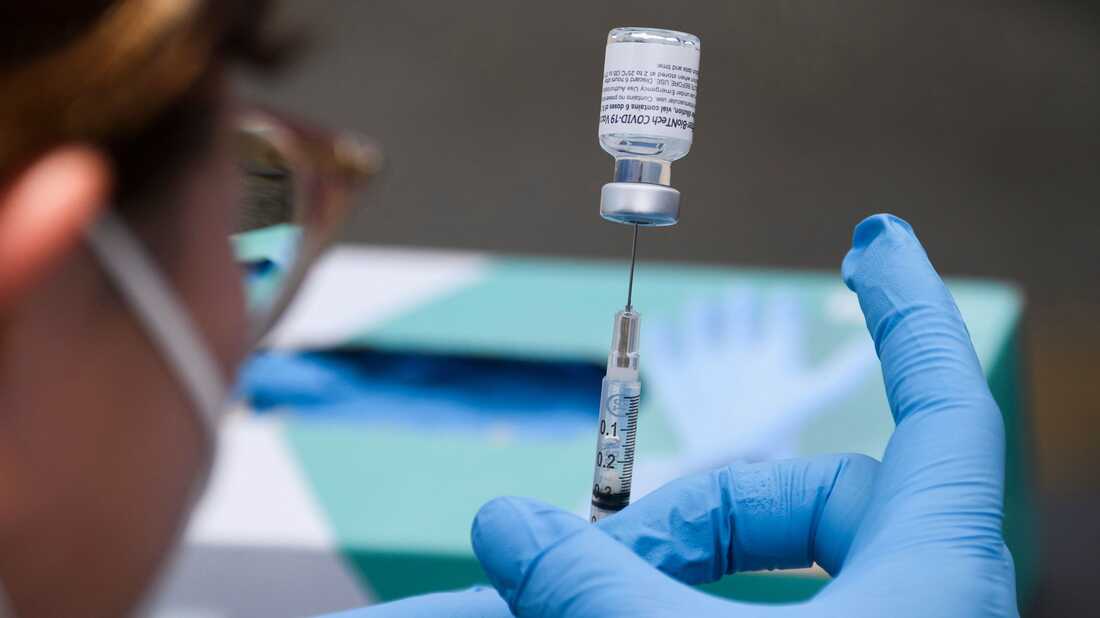
The CDC recommended that people with compromised immune systems have a third vaccination with either the Pfizer BioNTech or Moderna vaccine. The move follows FDA approval for such use a day earlier.
Patrick T. Fallon / AFP via Getty Images
Hide caption
Toggle caption
Patrick T. Fallon / AFP via Getty Images
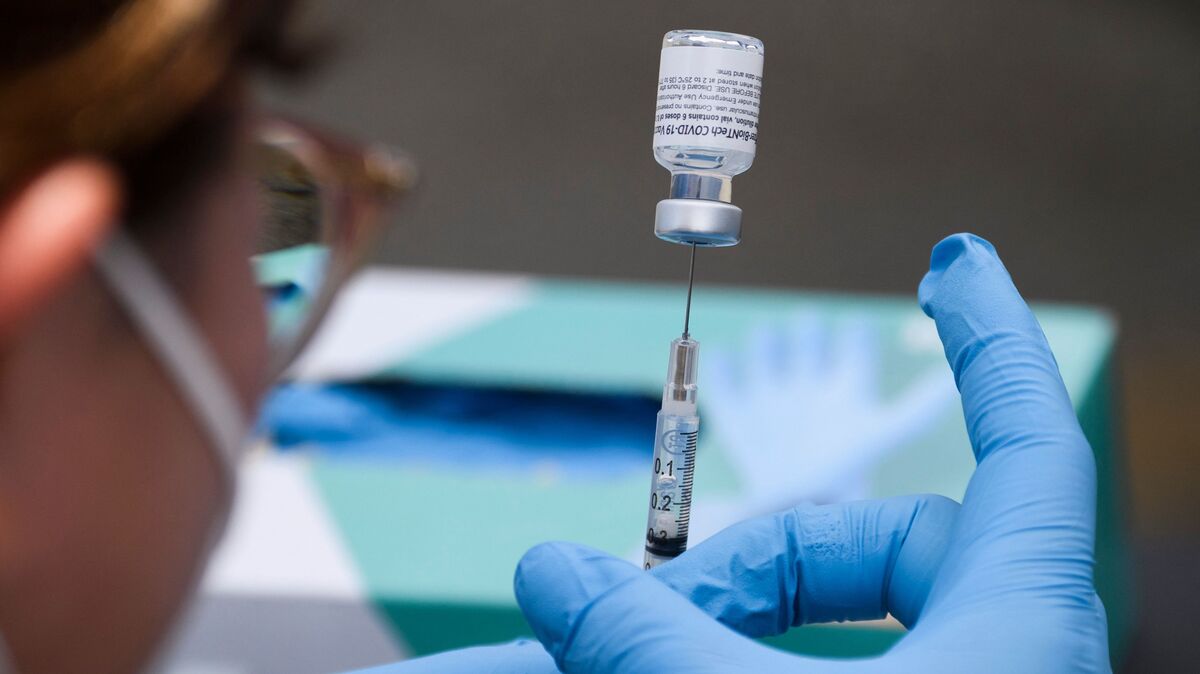
The CDC recommended that people with compromised immune systems have a third vaccination with either the Pfizer BioNTech or Moderna vaccine. The move follows FDA approval for such use a day earlier.
Patrick T. Fallon / AFP via Getty Images
The Centers for Disease Control and Prevention officially recommend that people with compromised immune systems receive a third vaccination with either the Pfizer BioNTech or Moderna vaccine.
It comes hours after a unanimous vote by an advisory panel on Friday to recommend the guidelines and less than 24 hours after the Food and Drug Administration approved such use.
Vendors generally await a CDC recommendation on vaccine use, even if the FDA has approved or approved a vaccine.
Immunocompromised people make up about 2.7% of US adults, or about 7 million people. They are more likely to get sick with COVID-19 and have a higher risk of prolonged coronavirus infection and transmission. They also have a lower antibody response to the initial vaccination schedule and are more likely to transmit the virus to household contacts, according to studies submitted to the advisory committee by CDC officials.
People with conditions that weaken the immune system or who are taking treatments that suppress immunity are also much more likely to have a breakthrough infection than people of normal health. A US study shows that 40% to 44% of breakthrough hospital admissions occur in immunocompromised people.


Studies have also shown that the vaccine is less effective in these people, ranging between 59% and 72%. In comparison, it is 90 to 94% in people without serious immunodeficiency. People who have received a solid organ transplant have the lowest immunity on the standard vaccination schedule.
The agency tells people about it the following conditions should take a third dose:
- You will receive active cancer treatment for tumors or blood cancer.
- You have had an organ transplant and are taking medication to suppress your immune system.
- have had a stem cell transplant or are taking medication to suppress the immune system in the past two years.
- Moderate or severe primary immunodeficiency (such as DiGeorge syndrome or Wiskott-Aldrich syndrome).
- Advanced or untreated HIV infection.
- Active treatment with high-dose corticosteroids or other drugs that may suppress your immune response.
The recommendation for the Moderna vaccine is limited to adults aged 18 and over, as this vaccine has not yet been approved for adolescents. This approval is expected by the FDA in the next few weeks. The Pfizer vaccine is approved for adolescents 12 years and older and adults.
People with other medical conditions are not advised to receive an additional dose at this time. “This would not include residents of long-term care facilities, people with diabetes, people with heart disease – these types of chronic conditions are not the intent here,” said Dr. Amanda Cohn from the CDC. Additional studies on how long immunity lasts in healthy people are underway and will determine the timing of additional doses for the general population, both FDA and CDC officials said.
The CDC said attempts should be made to match the type of additional dose that was used in the original shots someone receives. However, if this is not possible, an additional dose with the other vaccine is allowed. According to the data reviewed by the committee, the additional dose should be administered at least 28 days after the end of the primary series.
On Thursday, the FDA said the agency could not extend approval for an additional dose of the Johnson & Johnson vaccine due to insufficient data. Representatives from both agencies said they were “actively involved” in determining the best course of action for recipients of the Johnson & Johnson vaccine.
In a presentation to the committee, Dr. Kathleen Dooling of the CDC advised that immunocompromised people, including those receiving an extra dose, should continue to follow preventive measures, including wearing a mask and keeping 6 feet away from others they do not live with, and avoiding crowds and bad ventilated interiors until your doctor advises otherwise.
Close contacts of immunocompromised people should be strongly encouraged to get vaccinated against COVID-19, she said.
Separately, the CDC said that around 1.1 million people who do not necessarily have immune system dysfunction have already received one or more extra doses of the Moderna or Pfizer vaccines. About 91,000 have received one or more extra doses of the Johnson & Johnson vaccine.
The CDC recommendation comes during an intense new climb powered by the Delta variant. Since July 1, the number of confirmed cases has increased by 700%, according to the CDC.
According to the agency, new experimental and observational data in adults suggest that an additional dose of COVID-19 vaccine with the Moderna or Pfizer vaccine may increase the antibody response and increase the proportion of immunocompromised patients who respond to the vaccine.

In her review of the evidence, Dooling said that small studies with an additional dose of the Pfizer or Moderna vaccine did not reveal any serious side effects. However, the standard dose of these vaccines has been associated with rare but serious side effects, including anaphylaxis and myocarditis and pericarditis in young adults. The effects of immune-compromising diseases on these rare events are unknown, she said.
Thank You For Reading!
Reference: www.npr.org
